Pfizer appears on track to become first fully-approved COVID vaccine
The United States may have its first fully-approved vaccination against COVID-19 before Labor Day.
Last month, Pfizer-BioNTech announced the U.S. government is likely to complete the approval process by the start of next month and grant full approval of their vaccine as a way of boosting the public's confidence in all of the COVID vaccines.
This plan is based on a recent report indicating that if the U.S. Food and Drug Administration (FDA) granted full approval of at least one of the vaccines, more people would express willingness to be vaccinated.
According to a poll conducted by The Kaiser Family Foundation, three of every 10 unvaccinated people said they would be more likely to get a shot with a fully approved vaccine.
The New York Times reports that final approval would likely play a major role in steering the public away from misinformation about the safety of vaccines and in providing clarity to legal issues about vaccine mandates.
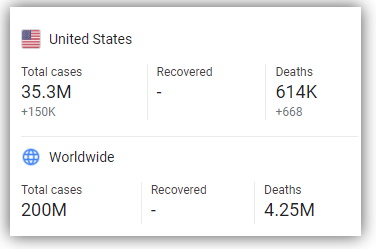
The FDA's decision to hasten the approval process in relation to Pfizer-BioNTech’s coronavirus vaccine comes as a number of communities in the United States experience a resurgence in the spread of coronavirus.
Trending News
Granting final approval of the Pfizer vaccine may also motivate a number of universities, hospitals, and other organizations to mandate inoculation.
Moderna, the second most widely used vaccine in the United States, requested final approval of its vaccine on June 1 and the company is still in the process of submitting data.
Johnson & Johnson, the third vaccine authorized for emergency use in the U.S., has yet to apply for full approval from the FDA.


